27+ pages thermal stability of group 2 sulphates 3mb. As we move down the group 2 the size of cations increases and sulphate is a big ion group according to its size. Thermal stability of group 1 sulphates. By continuing you agree to the use of cookies. Check also: sulphates and understand more manual guide in thermal stability of group 2 sulphates Thermal stability is the decomposition of compounds on heating.
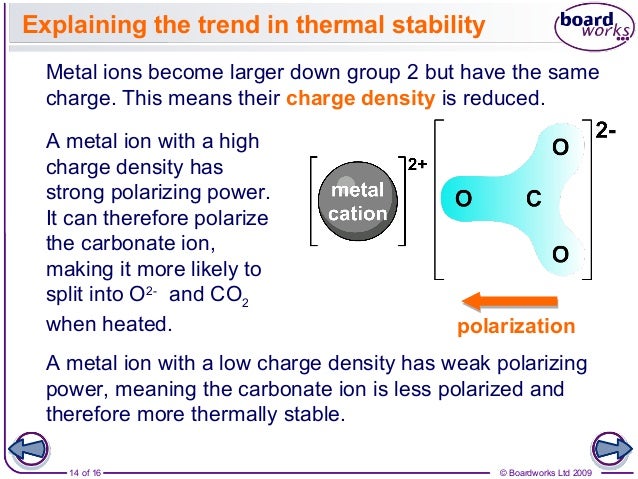
In group1it is found that the thermal stability of hydroxidescarbonatesnitrates sulphates etc. On going down the group2 polarising power also decreases and hence applying size factor stability of thermality increases.

Groups 1 And 2 Pounds 09 November 2020
| Title: Groups 1 And 2 Pounds 09 November 2020 |
| Format: ePub Book |
| Number of Pages: 229 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: September 2019 |
| File Size: 800kb |
| Read Groups 1 And 2 Pounds 09 November 2020 |
 |
In this video we want to explain the trends that we observe for thermal decomposition temperatures for Group 2 Metal Salts1.
As you go down the group the energy needed to break up the lattice falls as the positive ions get bigger. The thermal stability of a substance depends heavily on the structure of the substance which in turn is dependent on the bonds between atoms that hold that structure together. They can lower the energy needed to break a NO bond. MnSO 4 755 FeSO 4 537 CoSO 4 708 NiSO 4 675 CuSO 4 598 ZnSO 4 646 CdSO 4 816 PbSO 4 803 MgSO 4 895 CaSO 4 1149 and SrSO 4 1374C. More the strong bonding more thermal stability. MgCO3s MgOs CO2g Thermal decomposition is defined as the use of heat to break down a reactant into more than one product Lets use MgCO.
Trends In Group 2 Part 3 Chemical Properties
| Title: Trends In Group 2 Part 3 Chemical Properties |
| Format: eBook |
| Number of Pages: 274 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: July 2021 |
| File Size: 1.2mb |
| Read Trends In Group 2 Part 3 Chemical Properties |
 |
Trends In Group 2 Part 3 Chemical Properties
| Title: Trends In Group 2 Part 3 Chemical Properties |
| Format: ePub Book |
| Number of Pages: 313 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: April 2021 |
| File Size: 810kb |
| Read Trends In Group 2 Part 3 Chemical Properties |
 |

Inanic Chemistry Group 2
| Title: Inanic Chemistry Group 2 |
| Format: PDF |
| Number of Pages: 157 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: March 2019 |
| File Size: 2.2mb |
| Read Inanic Chemistry Group 2 |
 |

Total 1 Average 5 X2f 5 Redox Reaction In The Displacement Of Metals From Its Salt Solution Generally Metals Redox Reactions Solutions Reducing Agent
| Title: Total 1 Average 5 X2f 5 Redox Reaction In The Displacement Of Metals From Its Salt Solution Generally Metals Redox Reactions Solutions Reducing Agent |
| Format: PDF |
| Number of Pages: 349 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: April 2021 |
| File Size: 810kb |
| Read Total 1 Average 5 X2f 5 Redox Reaction In The Displacement Of Metals From Its Salt Solution Generally Metals Redox Reactions Solutions Reducing Agent |
 |

Thermal Stability Of Carbonates Of Group 1 And 2 Chemistry Notes Chemistry Thermal
| Title: Thermal Stability Of Carbonates Of Group 1 And 2 Chemistry Notes Chemistry Thermal |
| Format: eBook |
| Number of Pages: 289 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: April 2018 |
| File Size: 800kb |
| Read Thermal Stability Of Carbonates Of Group 1 And 2 Chemistry Notes Chemistry Thermal |
 |

Inanic Chemistry Group 2
| Title: Inanic Chemistry Group 2 |
| Format: PDF |
| Number of Pages: 147 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: January 2021 |
| File Size: 1.2mb |
| Read Inanic Chemistry Group 2 |
 |
Trends In Group 2 Part 3 Chemical Properties
| Title: Trends In Group 2 Part 3 Chemical Properties |
| Format: eBook |
| Number of Pages: 185 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: November 2018 |
| File Size: 1.3mb |
| Read Trends In Group 2 Part 3 Chemical Properties |
 |

Oxidation And Reduction In Electrolytic Cells A Plus Topper Electrolyticcellexperiment In 2021 Oxidation Chemical Changes Redox Reactions
| Title: Oxidation And Reduction In Electrolytic Cells A Plus Topper Electrolyticcellexperiment In 2021 Oxidation Chemical Changes Redox Reactions |
| Format: eBook |
| Number of Pages: 131 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: November 2020 |
| File Size: 1.8mb |
| Read Oxidation And Reduction In Electrolytic Cells A Plus Topper Electrolyticcellexperiment In 2021 Oxidation Chemical Changes Redox Reactions |
 |
Groups 1 And 2 Pounds 09 November 2020
| Title: Groups 1 And 2 Pounds 09 November 2020 |
| Format: ePub Book |
| Number of Pages: 192 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: April 2019 |
| File Size: 1.9mb |
| Read Groups 1 And 2 Pounds 09 November 2020 |
 |

The Elements Of Group Ii The Alkaline Earth
| Title: The Elements Of Group Ii The Alkaline Earth |
| Format: ePub Book |
| Number of Pages: 240 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: December 2021 |
| File Size: 1.35mb |
| Read The Elements Of Group Ii The Alkaline Earth |
 |
Trends In Group 2 Part 3 Chemical Properties
| Title: Trends In Group 2 Part 3 Chemical Properties |
| Format: PDF |
| Number of Pages: 244 pages Thermal Stability Of Group 2 Sulphates |
| Publication Date: May 2021 |
| File Size: 3.4mb |
| Read Trends In Group 2 Part 3 Chemical Properties |
 |
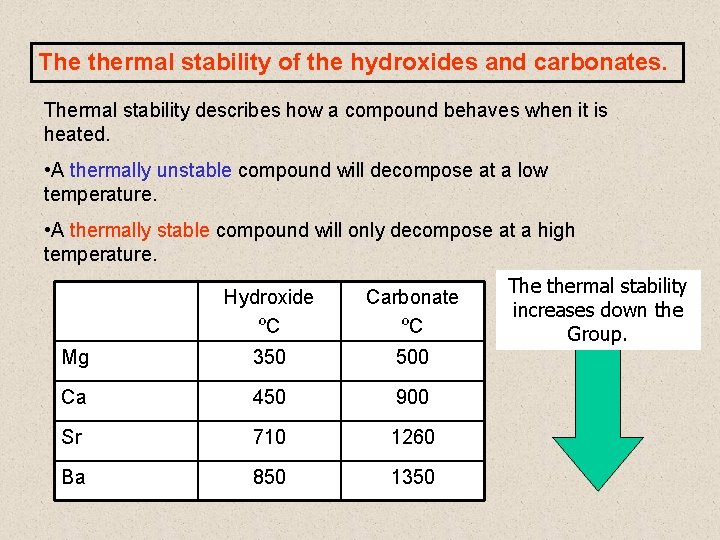
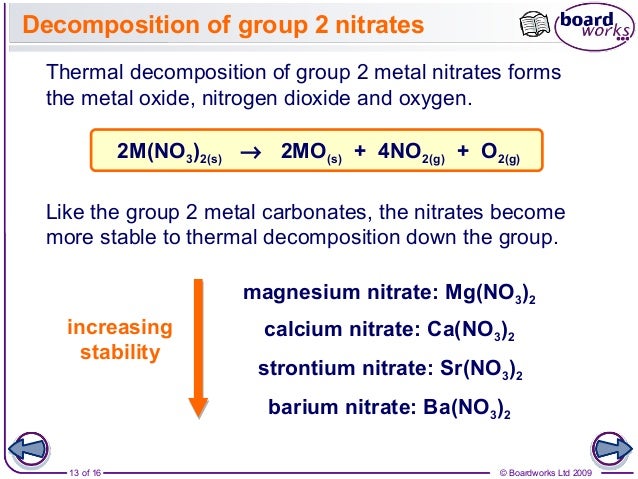
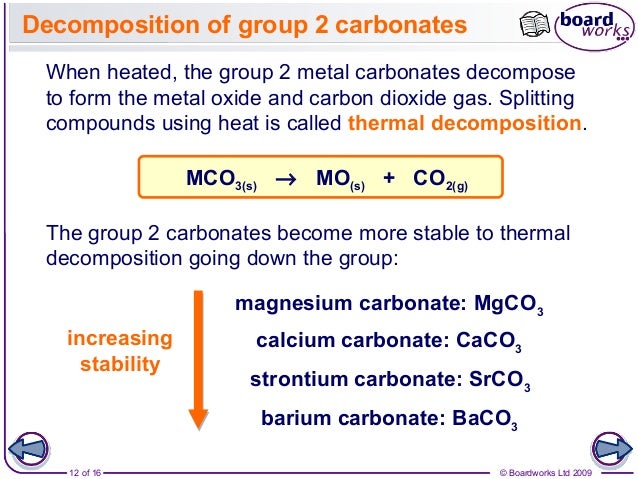
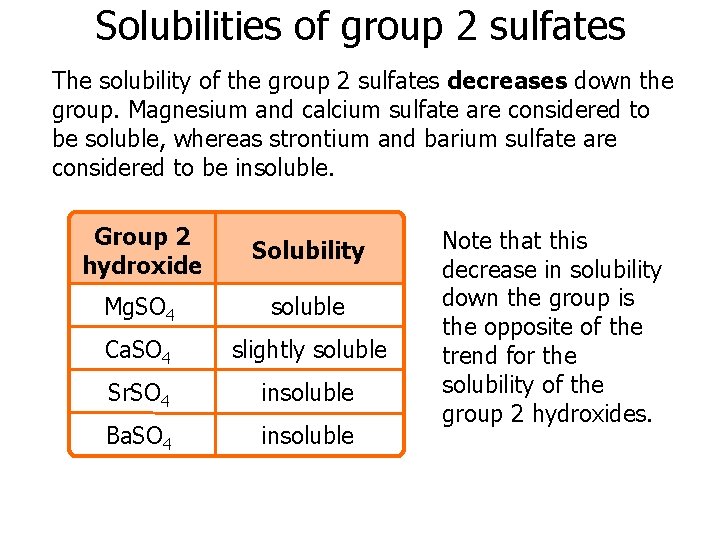
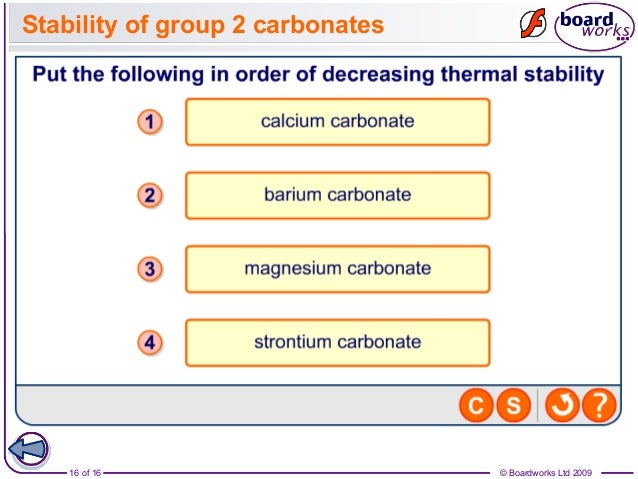
But since lattice energy is decreasing squarely and hydration energy is decreasing linearly the effect of decrease in lattice energy is higher than the effect of decrease in hydr. All the carbonates in this Group undergo thermal decomposition to give the metal oxide and carbon dioxide gas. Both carbonates and nitrates of Group 2 elements become more thermally stable down the group.
Here is all you need to know about thermal stability of group 2 sulphates The nitrates are white solids and the oxides produced are also white solids. But since lattice energy is decreasing squarely and hydration energy is decreasing linearly the effect of decrease in lattice energy is higher than the effect of decrease in hydr. Lets use MgCO 3 as an example. Trends in group 2 part 3 chemical properties trends in group 2 part 3 chemical properties oxidation and reduction in electrolytic cells a plus topper electrolyticcellexperiment in 2021 oxidation chemical changes redox reactions total 1 average 5 x2f 5 redox reaction in the displacement of metals from its salt solution generally metals redox reactions solutions reducing agent inanic chemistry group 2 trends in group 2 part 3 chemical properties It describes and explains how the thermal stability of the compounds changes as you go down the Group.
0 Komentar